
Virtually all men with metastatic prostate cancer will eventually develop metastatic castration-resistant prostate cancer (mCRPC), an advanced stage of the disease. With several treatment options for mCRPC, it is important to know when a class of therapy may not lead to a response.
AR-V7 by Epic Sciences is a liquid-biopsy test indicated for mCRPC patients who have failed at least one line of an AR-signaling inhibitor (ARSi) and are considering additional ARSis. AR-V7 is the only predictive and prognostic test for patients with mCRPC that:
ABOUT THE TEST
Patient Indications for AR-V7 Testing
The AR-V7 test is intended for use in patients with late-stage metastatic prostate cancer who:
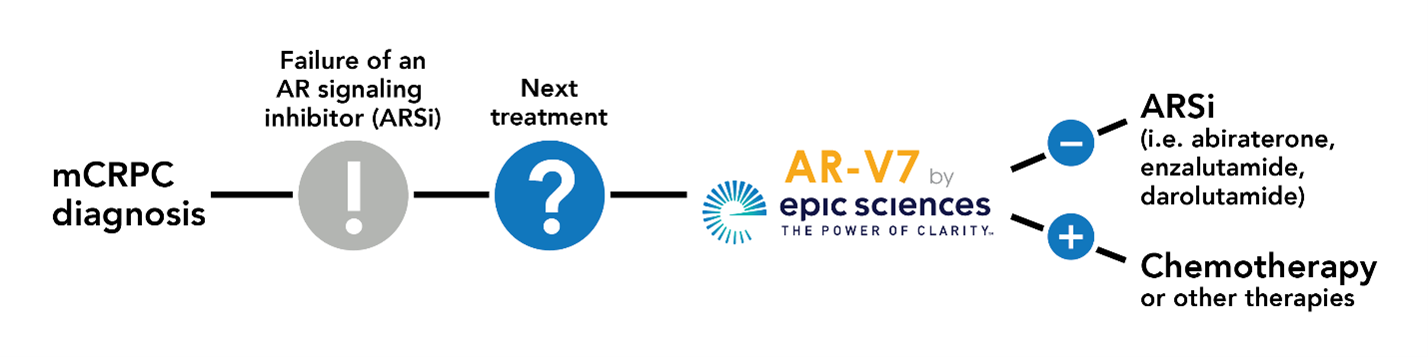
HOW IT WORKS
Epic Sciences’ technology can see, identify, and characterize nearly all the circulating tumor cells in a blood sample, and can predict their response or resistance to one class of drugs. Specifically, the AR-V7 by Epic Sciences test identifies patients who are likely to be resistant to androgen-receptor signaling inhibitor (ARSi) therapies such as abiraterone, enzalutamide, or apalutamide. Once alerted to the likelihood of drug resistance, physicians will be empowered to make alternative treatment choices that can improve outcomes and potentially reduce costs by avoiding ineffective treatment.
Affordability
Epic Sciences is committed to ensuring that all patients have access to medically necessary testing as determined by their healthcare provider. The Epic Support program assists patients and providers throughout the insurance coverage and billing process and offers financial assistance for qualifying patients.
Epic Support Program Overview
1
Epic test order received and sample released to the lab if insurance information is included
2
Insurance information used to estimate out-of-pocket (OOP) cost
3
Customer Success contacts patient if expected OOP >$100
4
Patient can complete Epic’s Financial Assistance Program (FAP) application at the time of test order (see link below) or Customer Success can assist with an application*
*Patients with government insurance are not eligible for Epic’s Patient Financial Assistance Program
If a patient experiences a financial hardship associated with their bill, Epic Sciences will work directly with the patient towards their complete satisfaction.
To check eligibility for Epic’s Patient Financial Assistance, please visit the online application here. For more information about our billing policies, please read the frequently asked questions.
Get Started
Beginning January 1, 2023 the AR-V7 test will be exclusively available through Epic Sciences. Paper test request forms and non-expired kits from Exact Sciences will continue to be accepted during the transition period. To download an AR-V7 by Epic Sciences test request form, click here.
To request new test kits or to schedule a meeting with a representative, please complete the form below. Have another question? Leave us a comment and we will be in touch shortly.
- Scher, H. I. et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker with Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol 2016;2(11):1441-1449. doi:10.1001/jamaoncol.2016.1828.
- Scher, H. I. et al. Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer. JAMA Oncol 2018 Sep 1;4(9):1179-1186. doi:10.1001/jamaoncol.2018.1621

